The International Congress on Innovative Approaches in Head and Neck Oncology (ICHNO) took place, for the seventh time, in Barcelona from 14 to 16 March. Anna-Bawany Hums participated in the interdisciplinary exchange of experts on behalf of oncgnostics GmbH.
Specialists from all over the World
Among the approximately 600 international participants, there were clinicians from various disciplines, as well as representatives from the research field. This year, the focus of the specialist lectures was on the presentation of results of current researches on Carcinogenesis: The spread of the disease, the application of different therapeutic approaches, and treatment strategies for head and neck tumours, as well as background and state of research in Radiation Oncology.

ICHNO 2019 in Barcelona
Interactive Event
The fact that the exchange between experts in Barcelona is very important was demonstrated by, for example, the so-called active tumour board. At the event, all participants were invited to discuss a real (but already closed) case. Using an online voting system, medical professionals were able to decide to use different treatment methods at certain points during the presentation of the case. The result reflected the implementation of various therapeutic approaches and the increasing use of new therapeutic strategies. However, there were also panel discussions and poster exhibitions.
Head and Neck Tumours in Research
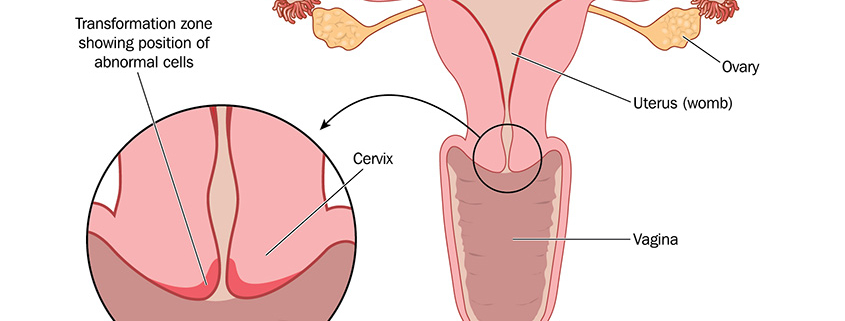
‘Such an interdisciplinary exchange opens up new perspectives on our work,’ states Anna-Bawany Hums. ‘In addition, the congress once again confirmed that biomarkers for the detection of malignant cells would offer many advantages for the affected patients, as a complement to current treatment strategies,’ continued the biologist with regard to the current research being conducted at oncgnostics, adding that ‘a cancer test for the detection of head and neck tumours such as the one we are developing requires only a saliva sample. For affected patients, this is a very gentle method for the clarification of cancer.’
Focus on the Oncological Patient

Fira de Barcelona – Veranstaltungsort der ICHNO 2019
What does supportive care mean? What services do patients need, for example, to cope better with the side effects of cancer? The focus of the congress was not only the
disease, but also the patient: ‘The topic was particularly vivid at an event in which a patient reported on her medical history. She talked about how doctors informed her about her disease, what helped her after therapy, and what she would have liked in doctor-patient communication. It was quite enlightening,’ Anna-Bawany Hums says.



 Dr Martina Schmitz faced a tight programme at four cities in only one week. From New Delhi to Pune, Coimbatore and finally Mumbai. Her itinerary started in the north and continued in the south of India, returning to the north again. Together with the other participants, our managing director visited medium-sized companies, and took part in various round tables and receptions, such as those organised by the Federation of Indian Chambers of Commerce and Industry (FICCI). Dr Martina Schmitz also visited two hospitals in Pune to have the Indian healthcare system explained to her.
Dr Martina Schmitz faced a tight programme at four cities in only one week. From New Delhi to Pune, Coimbatore and finally Mumbai. Her itinerary started in the north and continued in the south of India, returning to the north again. Together with the other participants, our managing director visited medium-sized companies, and took part in various round tables and receptions, such as those organised by the Federation of Indian Chambers of Commerce and Industry (FICCI). Dr Martina Schmitz also visited two hospitals in Pune to have the Indian healthcare system explained to her.
 Blamb/Shutterstock.com
Blamb/Shutterstock.com









